In biology, it is becoming increasingly important to analyze individual cells in the context of whole organs or tissues. So far, a standard approach has been to cut larger tissues into thin layers, study each section, and piece the information together into a 3D-model. This is a laborious process that often yields incomplete results.
The cells that make up our nervous systems possess long extensions that can reach throughout the entire body. It is extremely challenging to reconstruct such projections from small slices. Instead, new tissue clearing techniques can render opaque tissues transparent. When applied to complex tissues, e.g. the brain, tissue clearing techniques allow visualization ranging from entire nervous systems to individual cells and their extensions. They enable scientists to capture 3D-images of cells and tissues without the need for sectioning.
However, existing clearing techniques cannot remove the variety of pigments in the tissue, thus limiting their application to certain unpigmented organs such as the brain.
In a team effort, researchers from the Max Perutz Labs, the Medical University of Vienna, and the TU Wien (Vienna) and their collaborators have developed a new method that combines tissue clearing with the removal of a range of common pigment types. This new method, called DEEP-Clear, has been published in the international journal Science Advances.
A new way to image biomolecules in the nervous systems of a broad panel of species
The team discovered that a combination of different chemical treatments had a synergistic effect and allowed a fast depigmentation and tissue clearing. “Shortening the chemical processing preserves the tissues and organisms and increases the chance that relevant molecules and internal structures are retained”, explains Marko Pende, the developer of the clearing method, from the lab of Hans-Ulrich Dodt at the TU Wien and the Center for Brain Research (CBR).
We can image multiple organisms from different clades, ranging from mollusks over bony fish to amphibians. “These are just a few examples. We believe that the method applies to multiple organisms. We just haven’t tried it yet”, explains Prof. Hans-Ulrich Dodt.
Dr. Florian Raible’s team at the Max Perutz Labs has systematically explored which types of molecules can still be marked and detected in DEEP-Clear-processed samples. They have investigated species ranging from squids and worms to fish and salamanders. This work proved that DEEP-Clear is compatible with a range of different detection methods targeting a variety of important biomolecules and allowing to image specific proteins, DNA markers, and RNA in intact specimens.

Get a 3D-view of entire nervous systems using a light-sheet microscope built with a supercontinuum laser
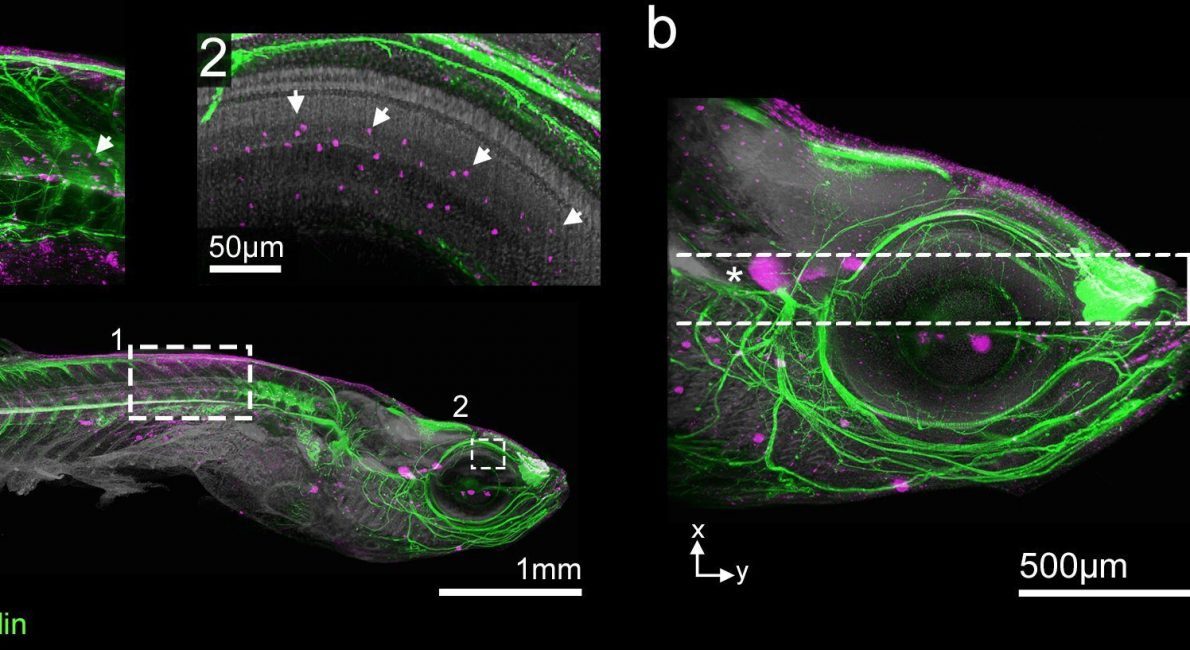
Once they have been processed using the DEEP-Clear method, you can image the transparent samples across scales: The team investigated very small details, such as contact points between neurons or individual clusters of dividing cells.
They used the latest generation of light-sheet microscopes equipped with a SuperK FIANIUM supercontinuum laser from NKT Photonics. The two-dimensional laser light is used to rapidly scan the whole sample, resulting in a full 3D-model created on the computer.
The mode properties of the laser determine the quality of the light-sheet illumination. The nicely collimated single-mode beam of the SuperK has a beam quality factor (M2) below 1.1, which enables results close to the theoretical limits. “Using a very thin light-sheet enables us to overcome a lot of optical limitations and allows us to generate high-resolution images, even from samples that are several millimeters thick“, says the designer of the microscopes, Dr. Saiedeh Saghafi from TU Wien.

“The combination of a SuperK VARIA tunable bandpass filter and the supercontinuum laser made it extremely easy to adopt the microscope for any stains labeled to the specimen. Essentially, you only have to dial a wavelength across the visible and near-infrared spectrum to match it with the optimal excitation of the labeling provided by our partners“
Marko Pende
The team expects that “DEEP-Clear” will become popular for tissue clearing. It will allow researchers worldwide to intensify molecular and cellular research in a variety of species that exhibit highly interesting – but poorly explored – neuroscientific features.
The supercontinuum laser offers the flexibility of choosing any wavelength and allows you to excite almost every common or emerging fluorescent label. “Having all colors from one fiber-coupled single-mode laser beam will make it very easy to keep our microscope well-aligned and sets us ready to image the variety of samples we would like to investigate in the future,” concludes Hans-Ulrich Dodt.
References
Pende et al., A versatile depigmentation, clearing, and labeling method for exploring nervous system diversity. Science Advances 6, eaba0365 (2020).
Meet the authors

Dr. Saiedeh Saghafi holds a Ph.D. in laser physics. Since 2008, she has been employed as a senior scientist at the Technical University of Vienna. In the Institute of Solid State Electronics, Department for Bioelectronics, she is designing a novel class of light-sheet microscopes that allow imaging of, so far, unreached sample sizes at a high resolution.

Marko Pende has pursued his Ph.D. project on new clearing and imaging techniques at the Medical University of Vienna and Vienna University of Technology (TU). In Prof. Dodt’s workgroup at the Center for Brain Research, the Section for Bioelectronics. He developed and applied the DEEP-Clear technique to generate high-resolution images from big volume samples of a variety of species, using the group’s custom light-sheet microscope.

Prof. Hans-Ulrich Dodt was appointed Professor of Bioelectronics at the Vienna University of Technology (TU) and runs a cooperation venture between the TU Vienna and the Center for Brain Research of the Medical University of Vienna. He is a physician and physicist who previously worked at the Max Planck Institute for Psychiatry in Munich, Germany. His research team develops and applies new optical methods for brain analysis, in particular, a new kind of microscopy (ultramicroscopy) to visualize neuronal networks in whole mouse brains or small organisms.

Dr. Klaus Becker holds a Ph.D. and a Diploma in Biology. He works as a research assistant at the Medical University Vienna and TU Vienna, where he develops deconvolution and data analysis algorithms for light-sheet microscopy to process the image data and perform statistics.