Using Supercontinuum lasers from NKT Photonics to provide tunable ultrashort pulse excitation, the group applied this approach across the scales from cuvette-based studies of molecular interactions [1] through rapid fluorescence lifetime imaging (FLIM) of fixed and live cells, e.g. for FRET readouts using fluorescent proteins [2,3], to preclinical molecular imaging using optical tomography techniques [4,5].
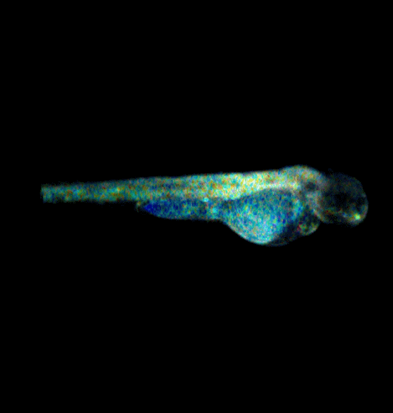
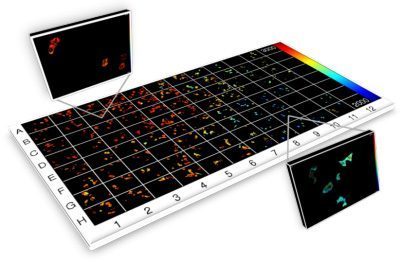
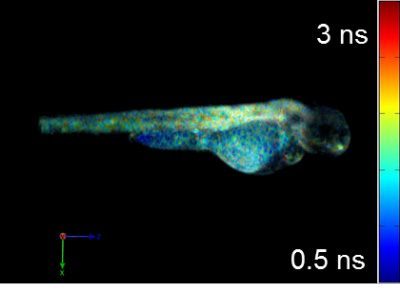
Current projects exploiting supercontinuum sources include automated medium-throughput FLIM implemented in multiwell plate readers to screen protein-protein interactions for drug discovery [6,7,8] and optical projection tomography (OPT), including FLIM OPT [9], of disease models such as zebrafish in which they can directly visualize key processes underlying disease and inflammation including tumor growth, angiogenesis, and cell migration.
Features and benefits
For the FLIM-based research, the team requires tunable ultrashort excitation pulses. Unfortunately, high peak power optical radiation is associated with photobleaching and phototoxicity. Picosecond pulses are therefore preferred to femtosecond pulses, making the supercontinuum source attractive for FLIM.
A single SC-400-6 unit can provide excitation of the most important fluorophores for the group’s work including CFP, YFP, GFP, mCherry, and further red fluorescent proteins as well as most dye-based fluorophores and some endogenous fluorophores including flavoproteins and porphyrins.
This makes it an unrivaled cost-effective source for their research and fluorescence microscopy in general, particularly FLIM. The team also values the compact nature and portability of these sources, which enables them to be rapidly deployed across a range of experiments.


“We use fiber-laser-pumped supercontinuum sources from NKT Photonics across our research programs and we simply would not have been able to undertake most of this research without them.
For us, the flexibility conferred by having ultrashort pulses on tap, tunable across the visible and NIR spectrum, is incredibly empowering and has enabled us to explore many different kinds of fluorescence imaging modality and many potential applications of our technology with a wide variety of samples and labels.
While such radiation could have been obtained from other laser sources, it would have been prohibitively expensive to match the capabilities of a single supercontinuum source and unthinkable to replicate this capability in the number of instruments we have developed.”
Professor Paul French, Faculty of Natural Science, Imperial College London
Read more at Imperial Colleage London, Natual Sciences’ website.
References
[1] H.B. Manning et al., “A compact, multidimensional spectrofluorometer exploiting supercontinuum generation”, J. Biophoton. 1, 494–505 (2008)
[2] D. M. Grant et al., “High speed optically sectioned fluorescence lifetime imaging permits study of live cell signaling events”, Opt. Expr.15 15656 -15673 (2007)
[3] D. M. Grant et al., “Multiplexed FRET to monitor multiple signalling events in live cells”, Biophysical Journal: Biophysical Letters 95 (2008) L69-L71
[4] J. McGinty et al., “Fluorescence Lifetime Optical Projection Tomography”, J Biophotonics 1, 390-394 (2008)
[5] J. McGinty, V. Y. Soloviev, K. B. Tahir, R. Laine, D. W. Stuckey, Joseph V. Hajnal, A. Sardini, P M.W. French, and S. R. Arridge, “3-D imaging of Förster resonance energy transfer in turbid media by tomographic fluorescence lifetime imaging”, Opt. Lett. 34, 2272-2274 (2009)
[6] C. B. Talbot et al., “High speed unsupervised fluorescence lifetime imaging confocal multiwell plate reader for high content analysis”, J. Biophoton. 1, 514–521 (2008)
[7] S. Kumar et al., FLIM FRET technology for drug discovery: automated multiwell plate high content analysis, multiplexed readouts and application in situ”, ChemPhysChem 12 ( 2011), 627-633
[8] D. Alibhai et al., “Automated fluorescence lifetime imaging plate reader and its application to Förster resonant energy transfer readout of Gag protein aggregation”, J. Biophotonics 6 (2012) 398-408,
[9] J. McGinty et al., “In vivo fluorescence lifetime optical projection tomography”, Biomed. Opt. Expr. 2 (2011) 1340-1350